What is the meaning of Equilibrium?
An equilibrium represents a state in a process when the observable properties such as colour, temperature, pressure, concentration etc do not show any change. The word equilibrium means ‘balance’ which indicates that a chemical reaction represents a balance between the reactants and products taking part in the reaction. The equilibrium state is also noticed in certain physical processes such as the melting point of ice at 0℃, both ice and water are present at equilibrium. In the case of physical processes such as the melting of solid, dissolution of salt in water etc., the equilibrium is called physical equilibrium while the equilibrium associated with chemical reaction is known as a chemical equilibrium.
Equilibrium in Chemical changes
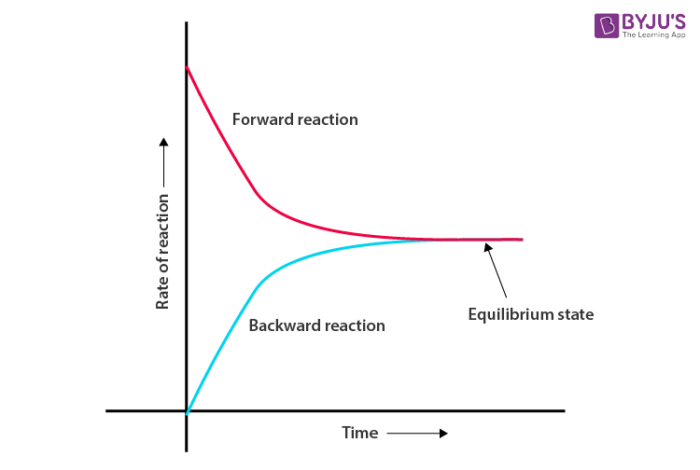
The chemical equilibrium in a reversible reaction is the state at which both forward and backward reactions occur at the same speed. The stage of the reversible reaction at which the concentration of the reactants and products do not change with time is called the equilibrium state. Or The state in which the measurable properties of the system such as pressure, density, colour or concentration do not undergo any further noticeable changes with time under a given set of conditions is said to be a state of equilibrium.
For more information Wikipedia